Study by blood doctors a breakthrough for haemophiliacs
- Wits University
A Wits University haematologist is the lead author in research set to revolutionise the treatment of haemophilia, a genetic blood disorder.

The HAVEN 3 study found that a new type of protein, emicizumab (trade name: Hemlibra), can be administered subcutaneously [under the skin], rather than intravenously [in the veins], and that it does not cause an immune response which prevents blood from clotting.
People with haemophilia bleed spontaneously or following trauma. The most common sites of bleeding are into joints, but bleeds can also happen into any tissue in the body, including the muscles, brain, or eyes. Many bleeds can be life-threatening, organ-threatening or fatal. Haemophilia affects only males because it is an X-linked condition, i.e., males have one X chromosome, whereas females are obligate carriers as they have two X-chromosomes.
Haemophilia impairs the body's ability to make blood clots, the process needed to stop bleeding. Haemophiliacs are born missing a protein called clotting factor and people who lack clotting factor VIII (FVIII) have haemophilia A.
To treat haemophilia A, the missing clotting factor VIII must be replaced. The replacement therapy is given prophylactically [as a preventative measure] to prevent spontaneous bleeds. However, there are two challenges:
- FVIII replacement is done intravenously and many patients – especially children – have difficulty accessing veins
- The FVIII may elicit an immune response in which antibodies are formed towards the replacement FVIII, thus rendering the replacement useless and incapable of stopping the bleeding.
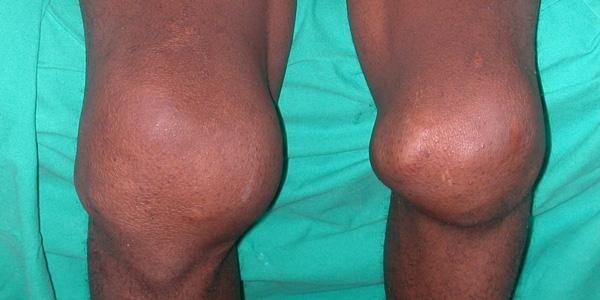
In the multi-centre, global HAVEN 3 study, patients aged 12 years and older were given a skin [subcutaneous] injection of new protein called emicizumab, which performs the same function as the missing FVIII.
The injections were administered weekly in one group, every second week in another group, and then the patients’ number of bleeds measured when compared to those not receiving emicizumab.
“Subcutaneously administered emicizumab reduced the bleed rate by 96% in those getting weekly emicizumab and by 97% in those receiving fortnightly emicizumab when compared to those not receiving emicizumab,” says Professor Johnny Mahlangu, clinical haematologist and lead author of the paper published in the New England Journal of Medicine on 30 August 2018.
Mahlangu is head of the School of Pathology in the Faculty of Health Sciences at the University of the Witwatersrand and head of the Haematology Diagnostic Section in the Department of Molecular Medicine and Haematology at the University.
Furthermore, 56% of patients receiving weekly emicizumab and 60% of those receiving fortnightly emicizumab did not experience any bleeding events whilst receiving emicizumab. For patients already on prophylaxis, there was 68% reduction in bleed rate on emicizumab when compared to the normal FVIII prophylaxis.
“Hemlibra [the trade name of emicizumab] is a prescription medicine used to prevent or reduce the frequency of bleeding episodes in adults and children with haemophilia A with factor VIII inhibitors. In the HAVEN 3 study, Hemlibra showed a significant and clinically meaningful reduction in bleeds in people with haemophilia A without factor VIII inhibitors, while offering flexible subcutaneous dosing options,” says Mahlangu.
WATCH a video produced by the NEJM about this research.

