Towards herd immunity from Covid-19: Costing a vaccine strategy for South Africa
- Alex van der Heever, Imraan Valodia, Lucy Allais, Martin Veller, Shabir Madhi, and Francois Venter
This article examines and estimates the financial implications of a vaccine strategy with a goal of achieving herd immunity.
It takes account of the roll-out phases identified by the Department of Health and vaccine prices available in the public domain. It finds that such a programme is affordable and implementable.
The overall cost of all three phases of a vaccine roll-out in South Africa, based on the AstraZeneca (AZ) vaccine and Covax supplies, would be R7.4-billion — with the need to consider an additional R1.2-billion for nursing costs. This makes a total outlay of R8.6-billion.
On the high side, the total cost of using the rival Pfizer vaccine would be R15.2-billion, with the extra R1.2-billion for nurses resulting in an outlay of R16.4-billion.
In evaluating the economic merits of this outlay, consideration has to be given to the approximate loss of R389-billion in economic output that occurred due to Covid-19 in 2020, together with the dramatic deterioration in the fiscal position of government. Even the highest cost is roughly half the value of tax revenue lost on tobacco and alcohol in 2020, estimated at R35-billion (which includes all tax revenue lost and not merely the excise taxes).
Based on this, it would be a false economy to delay the implementation of a full-scale vaccination programme on the basis of affordability. It is also highly probable that many sources of funding outside of government can be mobilised to expedite implementation.
It is therefore crucial that any further delays to the vaccine programme be removed and it be given the highest priority in government and the country.
Context
South Africa lost approximately 8.2% of GDP (or R389-billion) in 2020 due to the Covid-19 pandemic. In large part this was the result of the Level 5 and Level 4 lockdowns beginning in March. The remainder is due to reduced demand for certain services both before and after the heavy lockdowns ended. These include, inter alia, restaurants, hotels, gyms and medical practices.
However, many high-risk settings are vulnerable to behavioural lapses as life appears to return to normal. Viral transmission therefore increases as people return to work, certain behavioural adaptations lapse and society tries to find a “normal” that includes human contact. As the virus continues to circulate it also mutates, posing the risk of new variants that are more transmissible, involving more cases of severe illness and death.
In the absence of herd immunity (where the population levels protected from infection or less infectious are sufficient to bring about an interruption in the chain of transmission of the virus between people and within the population) all countries will experience years of periodic surges, resulting predominantly from the swings and roundabouts of human behaviour and government interventions of various forms.
Of all public health responses, vaccines are among the most effective and permanent. As evidence of this, smallpox, poliomyelitis, hepatitis B, measles, tetanus, diphtheria (whooping cough) and pneumococcal conjugate vaccine have reduced the morbidity and mortality of infectious diseases worldwide.
Efficacious vaccines are therefore central to the management of many infectious and contagious diseases globally. Other infectious diseases, such as HIV and tuberculosis, are contained through early diagnosis and treatment, while cholera requires clean water.
In the case of Covid-19, vaccine development has taken on unprecedented proportions and has had unparalleled success in efficiency of development. Multiple vaccine candidates began development and trials on an expedited basis in the second quarter of 2020. To address the international crisis, many vaccines began production in anticipation of approval in late 2020 and early 2021.
For South Africa to derive advantage, however, it needs to implement a vaccination strategy at a population level. Without this there will be insufficient immunity to avoid any resurgence in the substantial residual susceptible population.
The achievement of herd immunity depends on approximately 70% of the population developing immunity against SARS-CoV-2 infection or reducing their infectiousness. However, the longer it takes to roll out a programme, the more times a country will face a significant resurgence with all the contingent economic and social harm.
The success of a vaccination programme for Covid-19 will therefore influence the time taken to achieve herd immunity. The longer it takes, the more damage to the economy and society.
Elements of a strategy
Vaccine procurement
Two approaches to accessing vaccines present themselves.
First, there is the Covax arrangement which seeks to pool funding for a number of countries and to guarantee that up to 20% of their populations are covered. An upfront deposit is paid into the pool equivalent to 15% of the value of the commitment a country makes to purchase.
As and when vaccines are approved as efficacious and safe, vaccine manufacturers are committed to making agreed doses available for distribution. This framework seeks to assist low-income countries to gain access to vaccines. Higher and middle-income countries such as South Africa have participated to cross-subsidise income-compromised countries that will have difficulties accessing vaccines through bilateral arrangements.
Second are bilateral arrangements which many countries have entered into to expedite vaccine availability through advance market commitments (AMCs). As vaccines were under development during 2020, to ensure availability in 2021, many countries paid deposits against the supply of designated doses for delivery once regulatory approval had been obtained.
The significant benefit for the purchaser is guaranteed access once the vaccine has been developed.
Once a vaccine has regulatory approval, however, it is relatively risk-free for a country to procure supplies based on vaccine availability. The AMCs may, however, indirectly serve to restrict supplies for countries seeking to obtain vaccines without such an arrangement – at least initially. Up to 8.6 billion doses had been committed by the end of 2020, the bulk of them to high-income countries. It is probable that most of these arrangements involved AMCs.
South Africa, which falls into the upper-middle-income country group, however, had no advance purchase commitments through bilateral agreement at the time of writing, other than the Covax facility (which is also a version of an AMC). Other upper-middle- and lower-middle-income countries have, nevertheless, made commitments to accessing 1.1-billion and 1.96-billion doses, respectively.
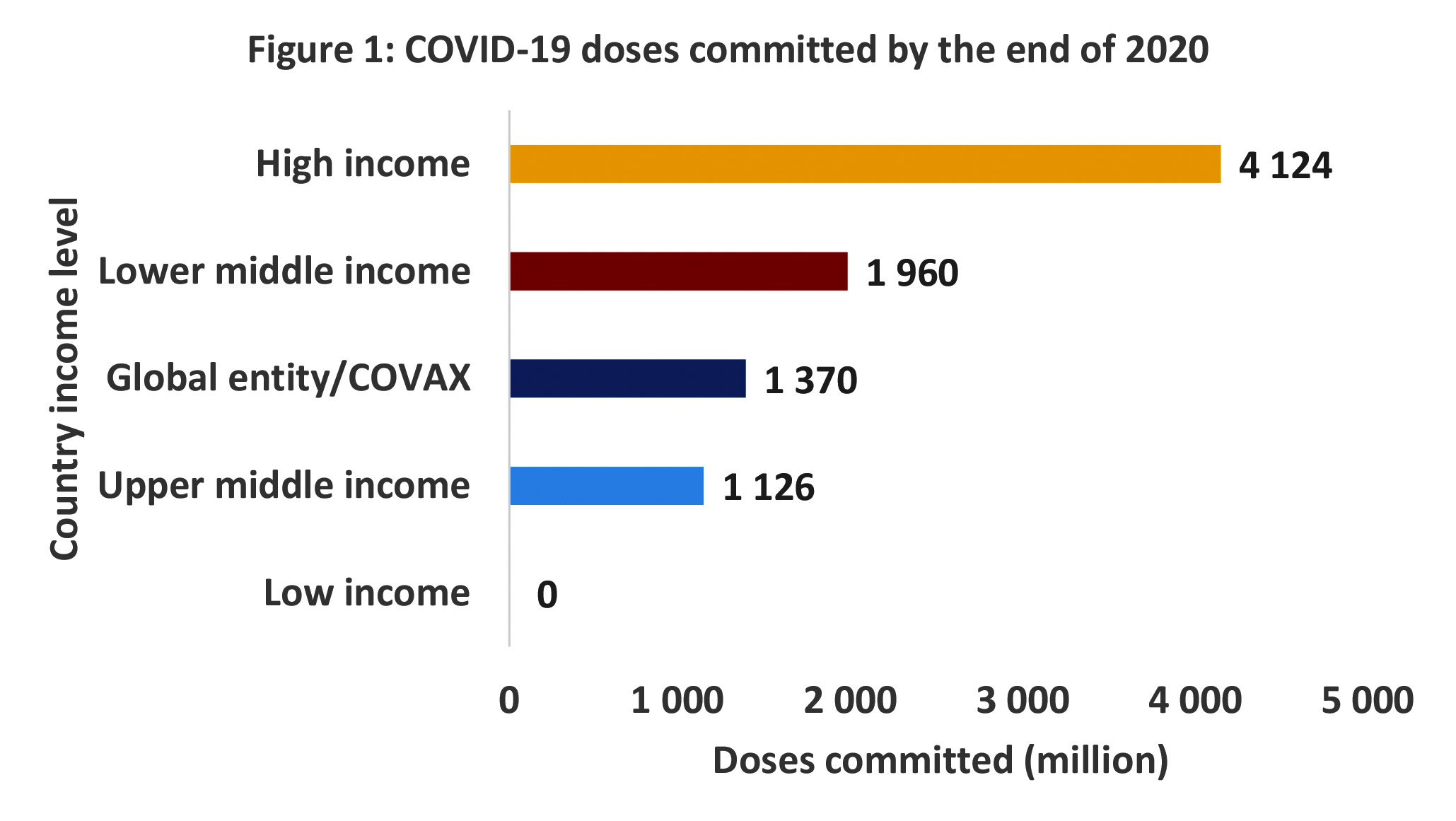
Source: Covid-19 launch and scale faster
By the end of 2020 South Africa had only committed to being part of the Covax arrangement, with a commitment equivalent to 10% of the national population, at a cost of R2.2-billion.
Despite falling into the upper-middle-income category of countries, South Africa chose not to enter into any bilateral agreements using AMC arrangements. This creates some risk that South Africa may face constraints in accessing vaccines in the early part of 2021 and may not be able to achieve herd immunity targets by the end of the year.
Indications are that the Covax facility will only deliver some vaccines around April 2021, exposing South Africa to an expected winter resurgence without vaccines as a preventive measure.
As a strategic objective, therefore, South Africa needs to urgently focus on high-volume bilateral agreements to supplement the Covax arrangement prior to April 2021 (i.e. in preparation for the anticipated winter resurgence). This would make available vaccines that have proven to be efficacious, safe, affordable and with manageable cold chain implications. It would, however, be optimal to finalise such agreements before the end of January 2021. Selected manufacturers need to be expressly encouraged to seek domestic regulatory approval where they have not applied as yet.
While this is recognised as an aspirational goal, it reflects the best possible outcome for managing the local epidemic.
Prioritisation
Government has outlined in broad terms a prioritisation strategy for rolling out a Covid-19 vaccine programme for South Africa. This involves three broad phases.
Phase 1 focuses on frontline health workers, including support staff and community health workers. The Department of Health (DOH) indicates this number at 1.25 million or 2.1% of the total population. At two doses for a complete regimen this would amount to 2.5 million doses.
Phase 2 focuses on high risk groups, including the aged and people with comorbidities. Broadly speaking, this could amount to about five million people or 8.3% of the total population. At two doses for a complete regimen this would require 10 million doses.
Phase 3 focuses on the rest of the adult population, excluding persons under the age of 16. The young are excluded as the vaccine trials did not cover them. Broadly speaking, this amounts to about 35.8 million people or 59.6% of the total population. At two doses for a complete regimen this would amount to 71.5 million doses.
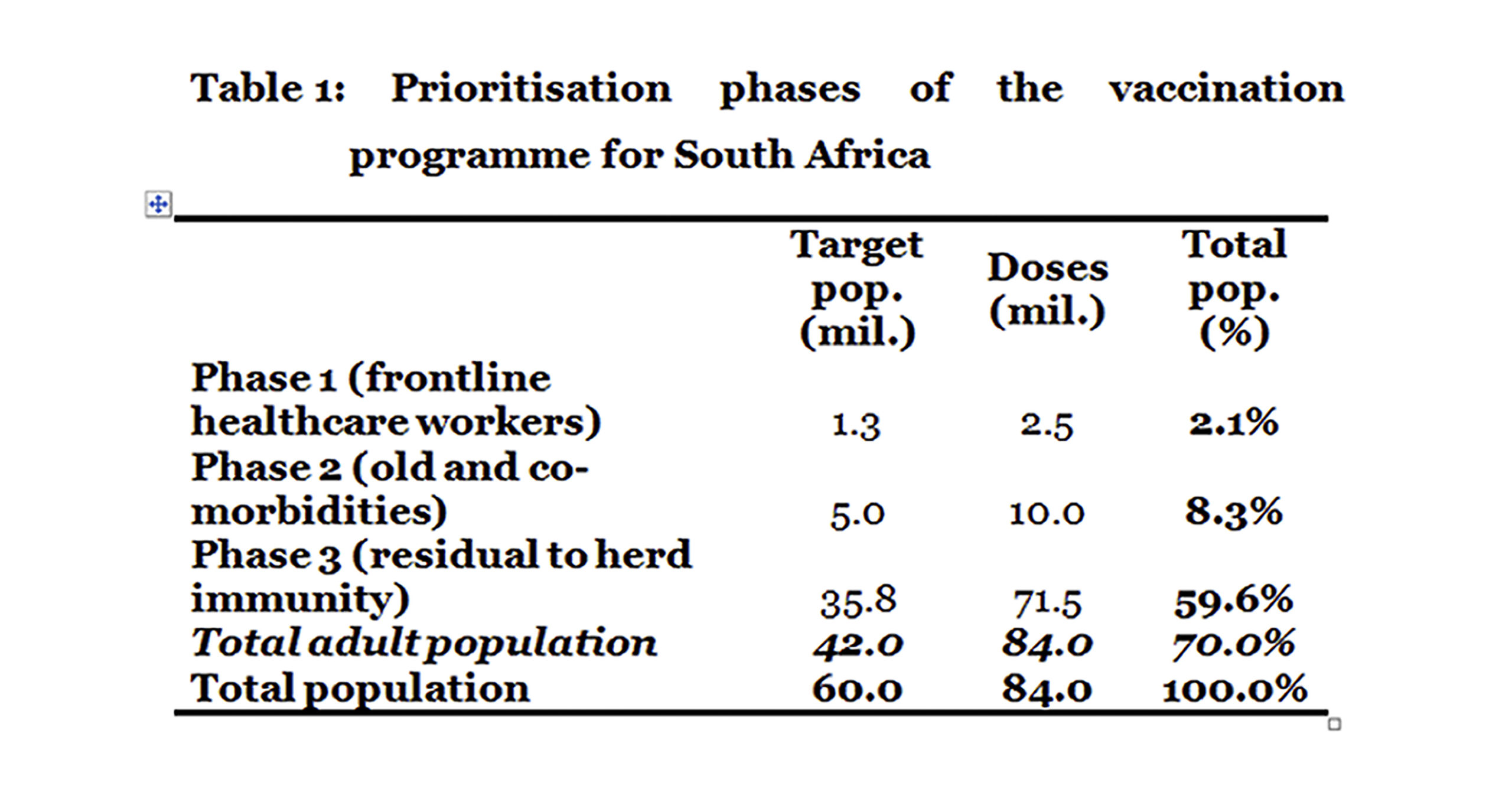
Assuming vaccine availability, phases 1 and 2 should be completed prior to May 2021, to reduce the impact of any winter resurgence on healthcare workers and those more vulnerable to severe illness.
An important question arises concerning the feasibility of vaccinating the residual adult population during 2021. From a strategic perspective, South Africa should pursue this as a goal even if it proves logistically impossible to finalise during 2021. As vaccinating this part of the population is required to achieve herd immunity and (hopefully) slows the emergence of mutations, it is imperative that every attempt is made to begin phase 3 in April/May and complete it if possible in 2021.
Again, it is recognised that this is aspirational. But nevertheless it offers an indication of the approach which best addresses the contingencies facing South Africa in 2021.
Financial feasibility
South Africa has the benefit of two large health systems, one tax financed and one through prepayment (contributions) to medical schemes. Together these systems achieve universal coverage through some form of prepayment. It will be important to fully utilise the capabilities of both systems to expedite the rollout of vaccines.
Two features of both systems will determine the speed and efficiency of any rollout. The first is funding the vaccine procurement. The second is the administration of the vaccine to the entire adult population. The former is evaluated here, while the latter is only addressed from a costing perspective.
To assess the financial implications of the proposed phased strategy of government, a range of vaccine candidates are analysed based on their pricing to date and the evidence of efficaciousness and safety. Their main characteristics are summarised in Table 2.
The three vaccines are: Pfizer, which requires two doses per regimen (this vaccine has been found to be efficacious and safe); Johnson & Johnson (J&J), which requires a single dose per regimen (this vaccine has not as yet been approved by a regulator and data for the single dose regimen is not yet available but is assumed to be sufficiently efficacious and safe for the purposes of this costing); and AstraZeneca (AZ), which requires two doses per regimen (which has been found to be efficacious and safe).
Of the three, both J&J and AZ have manageable cold chain implications as both can be refrigerated with a normal household fridge. The Pfizer vaccine, however, requires storage at -70o Celsius, which could prove problematic in South Africa as the required fridges tend to be available only in research facilities and to procure them at scale would probably prove difficult.
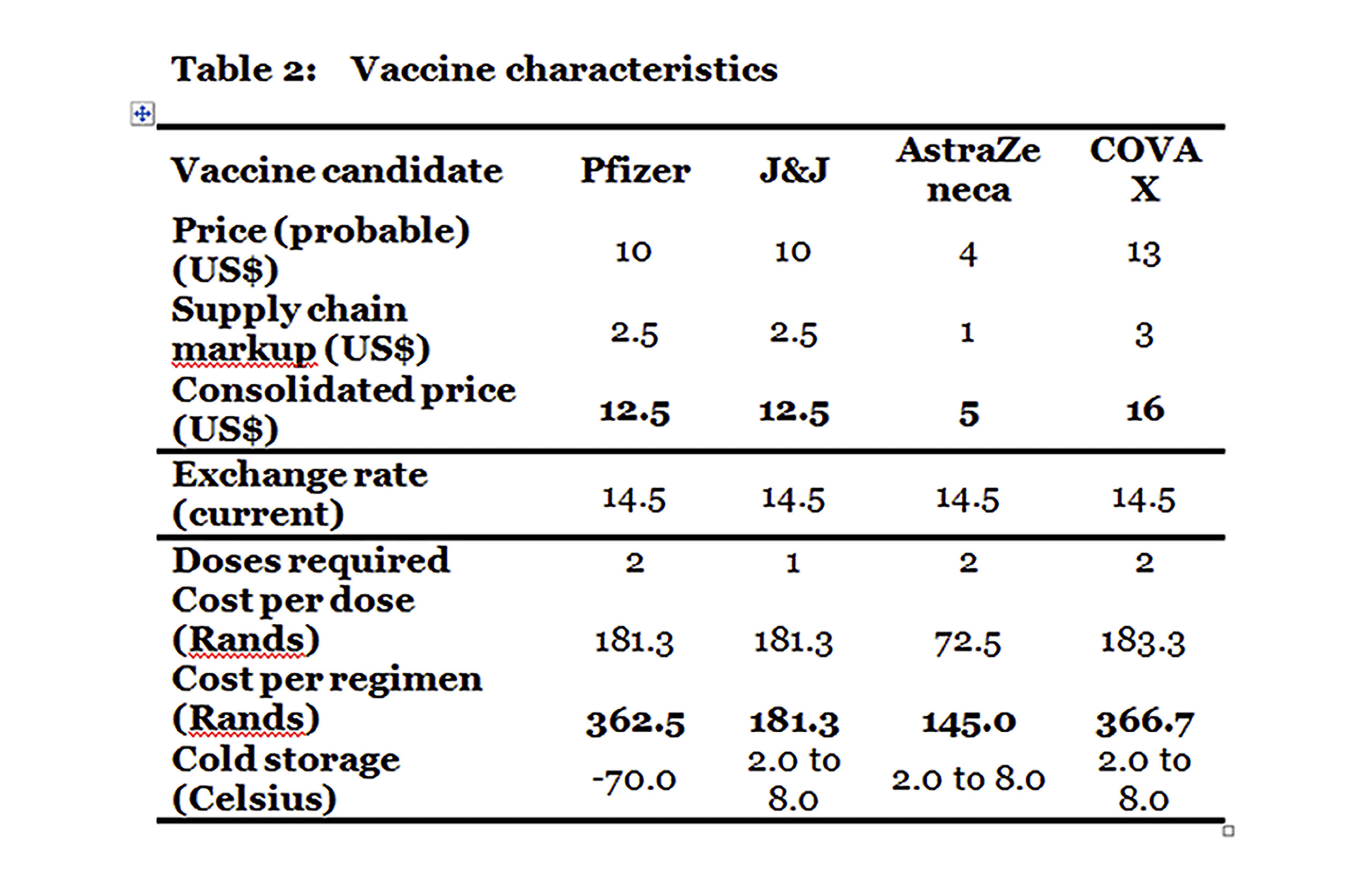
Including supply chain costs, assumed to be 25%, the cost per regimen (accounting for the number of doses) works out the lowest for AZ at R145 with J&J a close second at R181. The Pfizer vaccine is higher than both at R362.50.
When all the characteristics are taken into account, the AZ vaccine is a good candidate for wider rollout given its cost. Its only disadvantage relative to J&J is the need for two doses rather than one. However, it is not as yet known whether the single dose works or, if it does, what the relative durability will be to two doses of AZ.
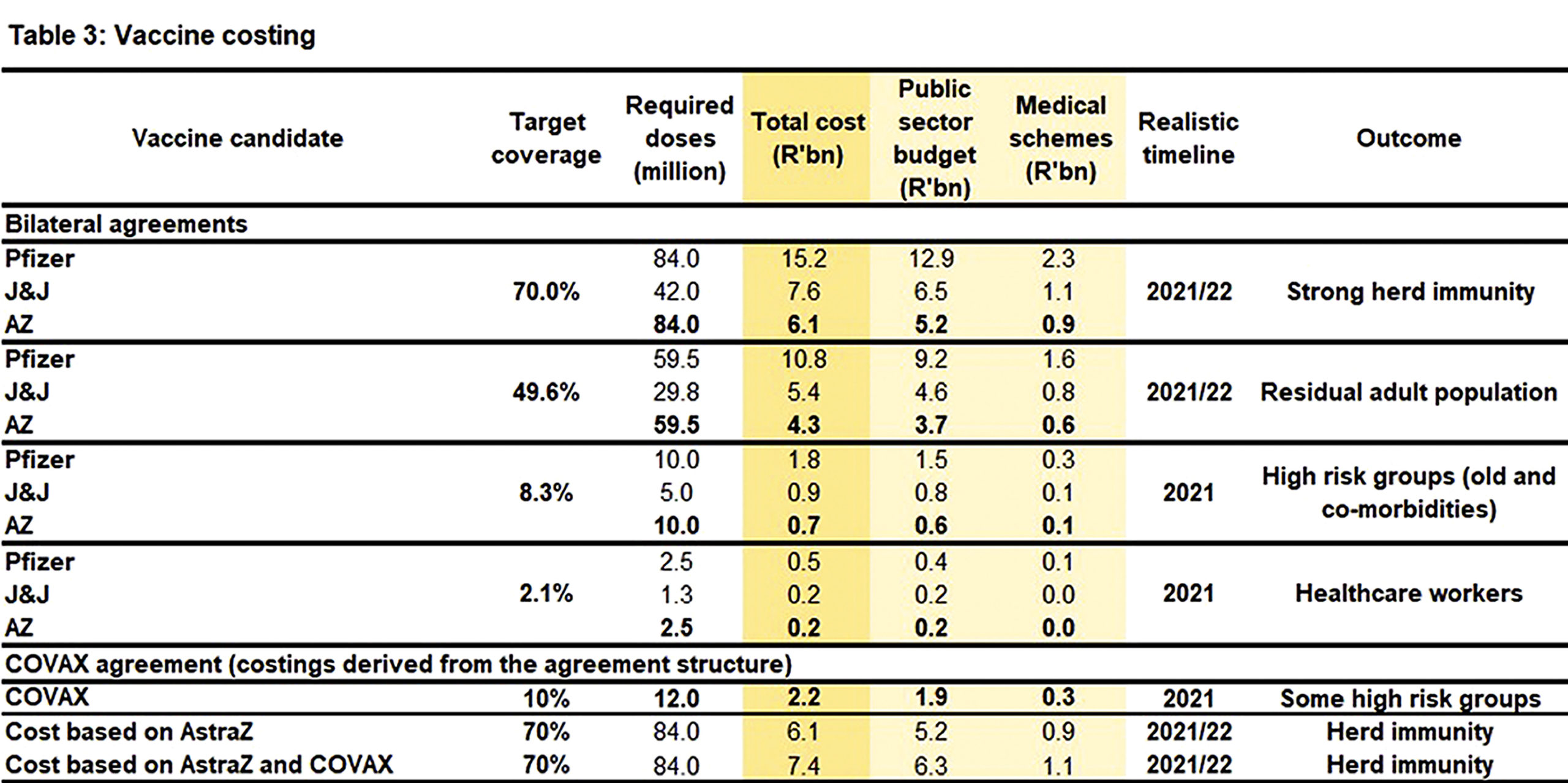
If South Africa adopts the DOH rollout and excludes the Covax arrangement, the following costing implications are implied (excluding supply chain costs): (Table 3):
For phase 1 the total cost ranges from R0.2 to R0.5 billion.
For phase 2 the total cost ranges from R0.7 to R1.8 billion.
For phase 3 the total cost ranges from R4.3 to R10.8 billion.
For herd immunity, the total cost ranges from R6.1 to R15.2 billion
Based on the DOH budget agreed with Covax, a higher cost per regimen (R366.70) is implied than with either AZ or J&J. The agreement actually comes in closer to Pfizer’s R362.50.
Based on this, if supply can be guaranteed through bilateral agreements it would make sense to limit exposure to the Covax arrangement.
If the Covax agreement is included in the mix, the total cost for herd immunity, based on the AZ vaccine, becomes R7.4-billion, i.e. a difference of R1.5-billion without Covax.
A further consideration is the implication of supply chain costs, which often include significant questionable mark-ups. It is not always easy to estimate these. For the sake of simplicity, a simple 25% mark-up has been assumed to provide a rough indication of the full cost of a programme to achieve herd immunity. For illustrative purposes, this adds R1.5-billion to the AZ-based programme with Covax in the mix, and R1.2 billion without Covax. It should be noted that these markups are included in the total costs discussed above and outlined in Table 3.
For completeness, it may be necessary to employ an additional nursing staff complement to avoid any diversion of the workforce away from frontline services. A rough indication of the nursing requirement assumes that one nurse can vaccinate roughly 26 people in a day. To vaccinate 40 million people over a 12-month period, assuming weekends and public holidays are used, will therefore require 8,851 staff nurses. Based on present salaries, this would translate into an annual cost of R1.227 billion.
While the cost of a vaccination programme appears relatively affordable for South Africa, the pandemic has severely affected government’s finances. Consideration therefore must be given to diversifying the revenue sources to remove or at least mitigate fiscal considerations as an obstacle to affordability.
A number of options are therefore raised here, together with an indication of possible prerequisites for their consideration.
First, South Africa’s medical schemes are in a position to finance all the vaccination costs for their members.
To ensure this happens, the Minister of Health has added the Covid-19 vaccine and all associated costs to the list of mandatory medical scheme benefits (referred to more commonly as prescribed minimum benefits or PMBs) that must be covered at full cost.
In vaccinating 40 million people, medical schemes will carry between R0.9-billion and R2.3-billion of the total cost through PMBs.
Medical schemes could make an advance payment into a facility/fund which would then facilitate access to vaccines without complications.
Second, as medical schemes achieved underwriting surpluses in 2020 due to the significant net drop-off in demand for healthcare services due to Covid-19, consideration can be given to a request that medical schemes donate a portion of their funds toward the purchase of vaccines for non-medical scheme members.
This arrangement would have to be on a voluntary basis as not all schemes may be in the same financial position. However, as medical schemes cannot by law pay for services for non-members, the Council for Medical Schemes would need to provide individual exemptions in terms of section 8h of the Medical Schemes Act.
The council has broad powers to exempt any person from any provision of the Act. An exemption should be granted where it conforms to the objects of the Act, which would be the case in this instance as the ending of an epidemic has implications for medical scheme members and non-members alike.
Third, large private corporations could agree on a voluntary basis to make donations toward the vaccination of non-members of medical schemes.
A single facility could be created into which the finances from the above three sources are paid. This could be the Solidarity Fund, or any other vehicle able to serve a similar purpose.
Putting it all together
Based on this analysis, the cost of a Covid-19 programme is far from prohibitive and could easily be financed by government on its own, private actors on their own or a partnership between government and private actors.
It is clear that the Covax facility cannot address South Africa’s goal of achieving herd immunity timeously. Bilateral approaches are therefore urgently required to expedite a national rollout programme to begin as early as possible. The three phases as proposed by the DOH appear reasonable.
The total cost of the first two phases of the rollout are negligible, irrespective of the vaccine chosen. Phase 1 would cost between R0.2-billion and R0.5-billion with a requirement for 2.5 million doses assuming two doses per regimen. Phase 2 ranges from R0.7-billion to R1.8-billion with a requirement for 10 million doses assuming two doses per regimen. In total, phases 1 and 2 require 12.5 million doses.
It should be noted that virtually all frontline healthcare workers are on medical schemes and the government need not fund their vaccines. (Note that Table 2 doesn’t reflect this as the split between the public sector budget and medical schemes is just a straight ratio of medical scheme members to the population dependent on the public sector.) The entire phase 1 is therefore an expense for private funds without any change to any law or public service configuration.
Phase 3, which takes South Africa to herd immunity, is also affordable, ranging from R4.3-billion to R10.8-billion. On the two-dose regime, 59.5 million doses would be required.
The overall cost of all three phases, based on the AZ vaccine together with Covax in the mix, would be R7.4-billion, with the need to consider an additional R1.2-billion for nursing costs, i.e. a total outlay of R8.6-billion.
On the high side, the total cost based on the Pfizer vaccine would be R15.2-billion, with an extra R1.2-billion for nursing, i.e. a total outlay of R16.4-billion.
The nurse contingency would depend on the operational approach adopted as some of the costs can be carried through the diversion of staff from current activities. However, overtime and additional nurses will carry a net cost implication.
It is worth noting that Level 5 lockdown cost South Africa in the region of R13-billion a day and has resulted in an estimated 8%-9% decline in gross domestic product for 2020. The accumulated debt of government is moving rapidly towards 100% of GDP and key industries are suffering harm from which they will take years to recover.
Furthermore, government is not in a position to provide social relief to people harmed by the pandemic due to its stretched resources.
Importantly, even the high estimate of the vaccine programme is roughly half the cost of the tax revenue foregone by government due to the alcohol and tobacco bans, which amounted to roughly R35-billion (taking account of all tax revenue sources and not just the excise taxes).
While the delay in expediting bilateral arrangements may work against South Africa, initiating negotiations are the test of what is feasible. As bilateral arrangements are plainly in the national interest, these should be expedited.
The need for an expedited programme also requires that trade-offs may need to be made between prices and speed of implementation. The costs of being slow potentially far outweigh the costs of a failure to reach herd immunity expeditiously.
It is worth noting at least two uncertainties that need to be accounted for in any strategy.
First, it may not be necessary to vaccinate 70% of the population to achieve herd immunity if natural infections induce durable immunity. The reported infection levels potentially undercount by several million people, a number that may increase significantly by the end of the current (second) surge. This could work in favour of an expedited strategy to achieve herd immunity.
Second, vaccine trials have mainly evaluated efficacy against Covid-19 illness, which is difficult to determine if the vaccine protects against asymptomatic infection. The high efficacy against mild Covid-19 illness, however, suggests that vaccines may have an impact on infection as well. Also, a vaccine may reduce infectiousness even if not preventing infection, which would contribute to inducing “herd immunity”.
These unknowns need to be taken into account as any vaccination programme is rolled out.
This article first appeared in the Daily Maverick/Maverick Citizen.
Alex van den Heever is Adjunct Professor at the Wits School of Governance
Shabir Madhi is Professor of Vaccinology: University of Witwatersrand and director: MRC Vaccines and Infectious Diseases Analytics Research Unit (VIDA)
Francois Venter is Deputy Executive Director of the Wits Reproductive Health and HIV Institute at the University of the Witwatersrand
Imraan Valodia is Professor of Development Economics at the University of the Witwatersrand
Martin Veller is Dean of the Faculty of Health Sciences at the University of the Witwatersrand and a Professor in the University’s Department of Surgery
Lucy Allais is Professor of Philosophy at the University of the Witwatersrand and director of the Wits Centre for Ethics

