How do Movement Paths Contribute to Migration Navigational Expertise in Plains Zebras?
Animals have complex movement pathways, exhibiting various scales of tortuosity that are often considered to contain a random element. Recent work, however, indicates that turns in animal pathways represent decision points, and that understanding turn incidence, extent and location is key to understanding how animals perceive and move through their environment. The movement of animals enables them to change their position in space, seeking favourable local or distant locations and thereby enhance their chances of survival. Movement paths encompass fundamental space changes ranging from sub-metre movements that can affect foraging success and predator evasion, to long-distance migrations, such as seen in plains zebras. Frameworks used to examine animal movements include random walk models (Hooten et al., 2017) that extend to models incorporating specific benefits, including, for example, the idea that randomness might enhance foraging success (Reynolds, 2018). Other studies show that movement taxis can occur as a response to features of the environment (Ahearn et al., 2017; Nicosia et al., 2017). Indeed, enhanced benefits resulting from movement choices are fundamental to explaining why animals should engage in energetically costly travel (Nisi et al., 2022; Gallagher et al., 2021), this being of relevance to the migration of plains zebras, how they navigate this migration, and for the co-migrating species that appear to be dependent on the plains zebras to undertake their migrations (link to video).
We aim to equip plains zebras with body- and head-mounted tags that will allow us to determine their position in space with high resolution, as well as to define how they allocate time to assessing their environment via head directionality (e.g., Gunner et al., 2021; Wilson et al., 2020). We propose that despite having lateralized eyes, the plains zebras will turn their heads towards objects of interest, that they will repeatedly scan the environment by turning their heads, and that significant body turn angles will correlate with prior head turns. We believe this will show that plains zebras scan their environment to determine beneficial and detrimental scenarios. If neither perceived benefit nor detriment is identified, the plains zebra will continue to move in a straight line; however, once either has been identified, the plains zebras will turn so that they either move towards beneficial space or away from detrimental space. The simple ‘scan’, then ‘ignore’, ‘move towards’ or ‘move away’ behavioural sequence provides a foundation for examining plains zebra movements and opens the possibility that we may be able to identify movement elicitors, such as landmarks used for navigating the migrations. Understanding what landmarks are important for navigation in the plains zebra is a crucial aspect of conserving the African Savannah Ecosystem. For example, if a specific large baobab tree is consistently used as a landmark for migrating animals, removal of this tree may be highly disruptive to the migration. Thus, in addition to knowing the migratory paths, understanding what the important landmarks on those paths are, will play a central role in managing human interventions in the migratory paths and minimizing the negative aspects of human activities. By understanding what features of the environment induce turning or visual inspection in the free-roaming plains zebra, along with an understanding of what the plains zebra can see, we can develop an understanding of the features of the landscape that are likely to be important to preserve on the various migration paths followed by plains zebras and co-migrating species.
Recent studies have shown that the speed of learning for the mental map within the navigation centre of the of the fly brain is dependent on turning, and that turning-based learning is dependent upon the release of the neurochemical dopamine (Taisz and Jefferis, 2022). Like all mammals (e.g., Dell et al., 2010), the plains zebra has a distinct dopaminergic neural system, located in the midbrain (see figure), and this system is known to be involved in both motor control and learning (e.g., Hefco et al., 2003; de Berket and Rutledge, 2014). Given the navigational expertise of the plains zebra, we can propose that the midbrain dopaminergic system, specifically the substantia nigra and ventral tegmental area nuclear complexes, might be enhanced in the plains zebra in comparison to phylogenetically related species, co-migrating species, and sympatric non-migrating species. Preliminary observations comparing the dopaminergic system of the plains zebra with that of the mountain zebra indicate that there is a quantitative enhancement of this system in the plains zebra (see Figure below), that may allow for better turning-based learning associated with navigational expertise. It is important to reveal whether the substantia nigra complex of the plains zebra is indeed more complex, and if so in what ways, than that of other relevant species, and this can be achieved with a comprehensive qualitative and quantitative neuroanatomical approach (done concurrently with other anatomical studies of the brain.
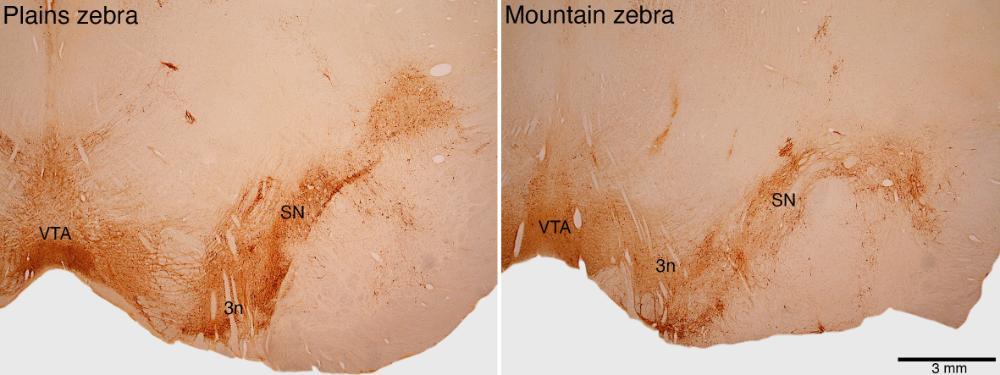
These low power photomicrographs reveal the cells of the midbrain dopaminergic system, stained by tyrosine hydroxylase immunohistochemistry, in the plains zebra and mountain zebra. The ventral tegmental area (VTA) and the substantia nigra (SN), separated by the passage of the oculomotor nerve (3n), form the main components of this system. The midbrain dopaminergic system in the plains zebra appears to have undergone a quantitative enlargement compared to the plains zebra.
Hypotheses:
(1) The plains zebras will follow the ‘scan’, then ‘ignore’/‘move towards’/‘move away’ behavioural sequence when making a decision regarding navigation through their environment.
(2) The midbrain dopaminergic system of the plains zebra will show aspects of specialization in comparison to relevant species.
Aim: To provide a neuroethological account of navigational decision making in the plains zebra, and to extend this to determining what landmarks they may be using in their decision-making process.
Specific Objective 1: By recording accelerometery, magnetometry and GPS data we plan to determine behaviour profiles and highly-resolve (dead-reckoned) movement paths, and to align these with potential landmarks in the environment.
Specific Objective 2: To determine, through qualitative and quantitative neuroanatomical analyses, whether the midbrain dopaminergic system of the plains zebra presents with any specific qualitative or quantitative enhancements that may help to explain their navigational expertise.
Methods:
Specific Objective 1: Up to 20 adult plains zebra (10 males, 10 females), inhabiting a large game reserve within South Africa, will be equipped with head- and body-mounted tags that record tri-axial acceleration at 40 Hz and tri-axial magnetic field intensity at 13 Hz using a real-time clock base (Wilson et al., 2008). The head-mounted tags will be affixed between the ears, while the body-mounted tag will be attached to the ventral part of a GPS collar. The GPS collar will be attached to the plains zebras to track their freely roaming movements in the game reserve (a location will be recorded every 5 minutes), and to be able to locate them at the end of the recording session (approximately 6 weeks) to retrieve the devices. All units will be calibrated to synchronize time and for local magnetic field conditions as well as hard and soft iron distortions (Gunner et al., 2021). After recovery of the tags, the individual head- and body-headings from all animals for all periods will be determined using established methods (Gunner et al., 2021) as well as times when ‘significant turns’ occurred. Three basic categories will be recognised: (i) just head turns, where the head turns but the body does not [‘head yaw’]; (ii) just body turns, where the body turns in a manner mirrored in time and extent by the head; and (iii) head turns followed by body turns, where a head turn is followed by a body turn within 3.5 s. The significant turns will then be localized with GPS co-ordinates and mapped onto a detailed map of the game reserve, including significant features, with spatial correlations aiming to determine what might be the landmarks used for the plains zebras to make a decision to turn.

This image shows how in donkeys a specifically designed head bridle (minus the bit) can be used to non-invasively attach appropriate head-mounted tags to the plains zebras for recording movements relevant to navigation.
Specific Objective 2: By employing immunohistochemical staining to reveal the cellular location of tyrosine hydroxylase (an enzyme involved in the production of dopamine), on sections of the brain, the midbrain dopaminergic system of plains zebras and other relevant species can be revealed. Both qualitative and quantitative analyses of this system can then be undertaken. Qualitative analyses will examine the presence or absence of specific portions of this system, as well as derive an impression of what regions might be enhanced or reduced in size (e.g., Dell et al., 2010). Quantitative analyses can provide estimates of total neuronal numbers, as well as the size of these neurons, that can be then compared across species (in relation to brain mass). These studies will provide a reliable answer as to whether the dopaminergic system in the plains zebra does contain certain specializations related to turning, learning, and navigation.
