The Anatomical Definition of a Cognitive Specialization
The observation that led to Project Plains Zebra was that the hippocampal formation of this species is the largest, both in actual size and in size relative to the brain, of all mammalian species that have been investigated to date (Patzke et al., 2015). As the hippocampal formation plays the central role in the cognitive neural navigation system, we believe it is important to study the hippocampal formation of the plains zebra, and to contextualize these findings in a phylogenetically relevant context, by studying closely related species (Equids), and an ecologically relevant context, by studying species that migrate with the plains zebra (Artiodactyls).
In addition, studies of other non-migrating Artiodactyls that inhabit similar environments need to be studied to further contextualize the results obtained. In addition to the brains of plains zebras (Equus quagga), those of the phylogenetically related mountain zebra (Equus zebra), domestic horse (Equus caballus) and domestic donkey (Equus asinus), will be investigated. Sympatric migrating species that will be examined include the blue wildebeest (Connochaetes taurinus), Grant’s gazelle (Nanger granti), Thomson’s gazelle (Eudorcas thomsonii), white-eared kob (Kobus leucotis), tiang antelopes (Damaliscus lunatus tiang) and Mongalla gazelles (Eudorcas albonotata). Non-migrating sympatric species to be studied include impala (Aepyceros melampus), waterbuck (Kobus ellipsiprymnus), nyala (Tragelaphus angasii), kudu (Tragelaphus strepsiceros), and eland (Taurotragus oryx).
By investigating this range of species, with the range of techniques outlined below, we will be able to comprehensively define the anatomical basis of what we believe to be a true cognitive specialization. These studies will provide important insights into how the enlarged neural navigation system of the plains zebra differs from other species, and how these differences might explain the exceptional navigational competency of the plains zebra.
Hypothesis: A variety of both overt and subtle variations in the structure, neurochemistry and connectivity of the hippocampal formation of the plains zebra will be observed. These variations will provide clues as to how the plains zebra uses this neural system to lead the great migrations of the African savannah.
Aims and Methods:
Specific Aim 1: Using standard neuroanatomical techniques and structural MRI, we aim to quantify the size, both absolute and relative, of the hippocampal formation, associated cortices and other brain nuclei related to spatial navigation. In combination with standard structural MR imaging, brains will be sectioned and stained with a range of techniques. This will allow the accurate analysis of the structure and volumes of the various components of the neural navigation system in all species targeted for study. Allometric analyses, with appropriate phylogenetically controlled statistical methodology, will be used to reveal variations in these components of the navigational system of the brain. In addition, by using immunohistochemical staining techniques we will be able to derive a detailed understanding of the internal architecture and neurochemistry of the neural navigation system in the species studied (refer to CR CA1 PZ vs MZ image). It is through these studies that we will be able to determine what features are common to all these species, and what features are specific, especially within the enlarged hippocampus of the plains zebra, that will contribute to our understanding of how the plains zebras lead the migrations.
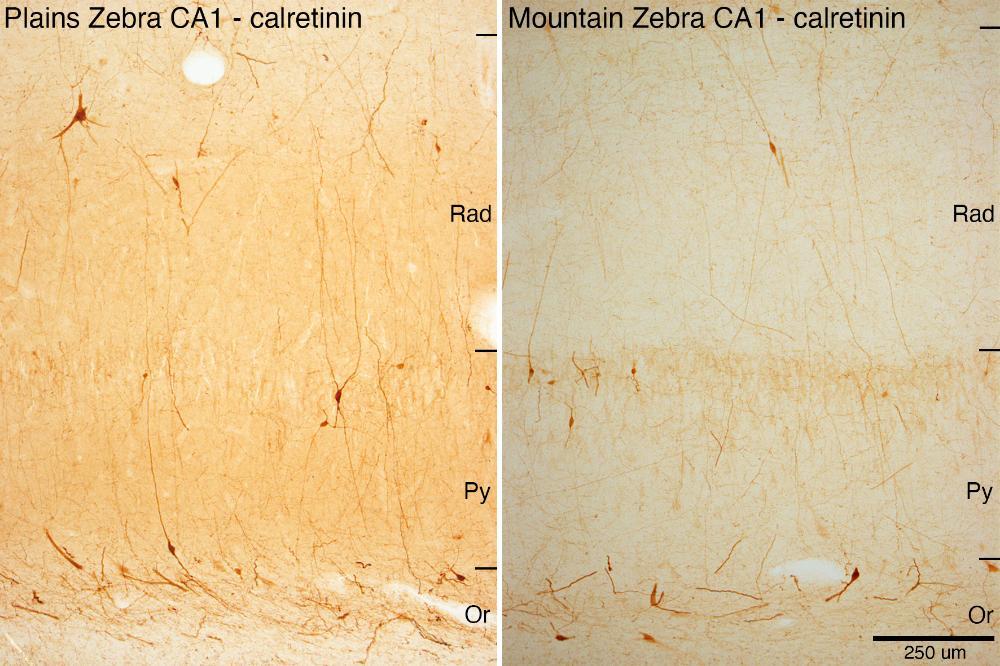
CR CA1 PZ vz MZ: These two photomicrographs show neurons within the cornu ammonis 1 region of the hippocampus in the plains zebra and mountain zebra immunostained to reveal the presence of the calcium-binding protein calretinin. While the number of stained neurons don’t appear to be obviously different, it is clear that the neuronal soma and dendrites are larger and possibly more complex in the plains zebra compared to the mountatin zebra. Rad – radiatum layer of hippocampus; Py – pyramidal cell layer of the hippocampus; Or – oriens layer of the hippocampus.
Specific Aim 2: Using immunohistochemical techniques and stereological analyses, we aim to quantify the various phases of adult hippocampal neurogenesis in the plains zebra and other species targeted for investigation. From the brains that will be sectioned for aim 1, appropriate evenly spaced sections will be immunostained for the following markers of adult hippocampal neurogenesis: Ki-67 to reveal the rate of cell birth, doublecortin to reveal the numbers of immature neurons (refer to Neurogenesis image), double-labelling of doublecortin and calbindin to reveal numbers of integrating neurons, caspase staining to reveal the rate of cell death, and neuropeptide-Y to reveal those neurons with specific neuroprotection. Stereological analysis, grounded against the number of dentate gyrus granule cells (measured using Nissl staining) will be undertaken to determine potential differences in adult hippocampal neurogenesis amongst species. This will provide comprehensive information about the process of adult hippocampal neurogenesis in the various species targeted for study and will reveal if this process is augmented in the plains zebra.
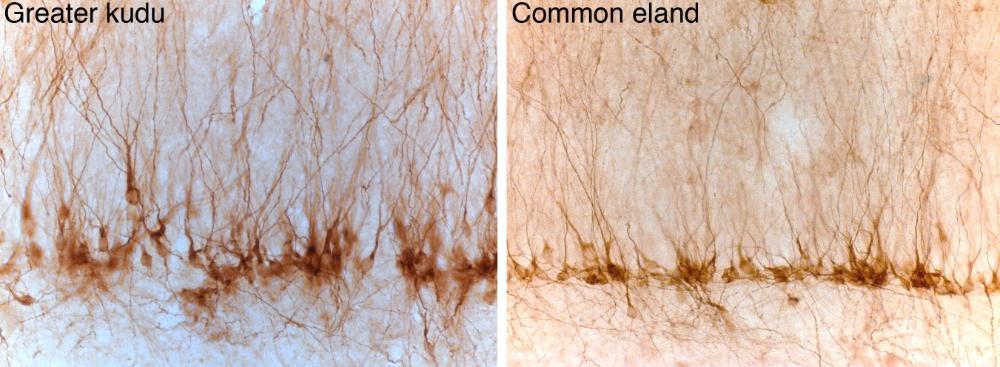
Neurogenesis image: These two photomicrographs shows immature neurons within the deeper aspect of the granule cell layer of the hippocampal dentate gyrus in the greater kudi and common eland immunostained for the doublecortin protein. These immature neurons develop in the adult hippocampus and help with the updating of memories and help in differentiating between similar memories. While we haven’t yet examined these in the plains zebra brain, they should be present and may show a real difference in their number, density, and morphology compared to non-migrating species.
Specific Aim 3: Using the well-established isotropic fractionator method (Herculano-Houzel and Lent, 2005; Herculano-Houzel et al., 2015a,b; Kazu et al., 2014), the number of neurons in the hippocampal formation of all species targeted for study will be determined. These will be compared to other neuronal numbers obtained through the processing of these brains (such as the numbers of cortical neurons), and then compared within the data obtained and to previously obtained data (Herculano-Houzel et al., 2015b), using appropriate allometric and statistical analyses.
Specific Aim 4: Using Golgi staining and neuron reconstruction analyses, we aim to quantify and compare the complexity of hippocampal neurons in the plains zebra and other targeted species. By applying standard Golgi impregnation methodology and neuron reconstruction analysis (Neurolucida, e.g., Jacobs et al., 2011), the complexity of individual hippocampal neurons can be established across the species (refer to golgi image). These will be compared across species within the study to determine if the plains zebra not only has the largest hippocampus, but whether, in addition, it has the most complex neurons (as observed for the elephant cerebellum in previous studies, Maseko et al., 2012, 2013; Jacobs et al., 2014). This is an important aspect of determining to what extent the larger hippocampus of the plains zebra is also specialised for processing of neural information and the cognitive complexity it can generate.
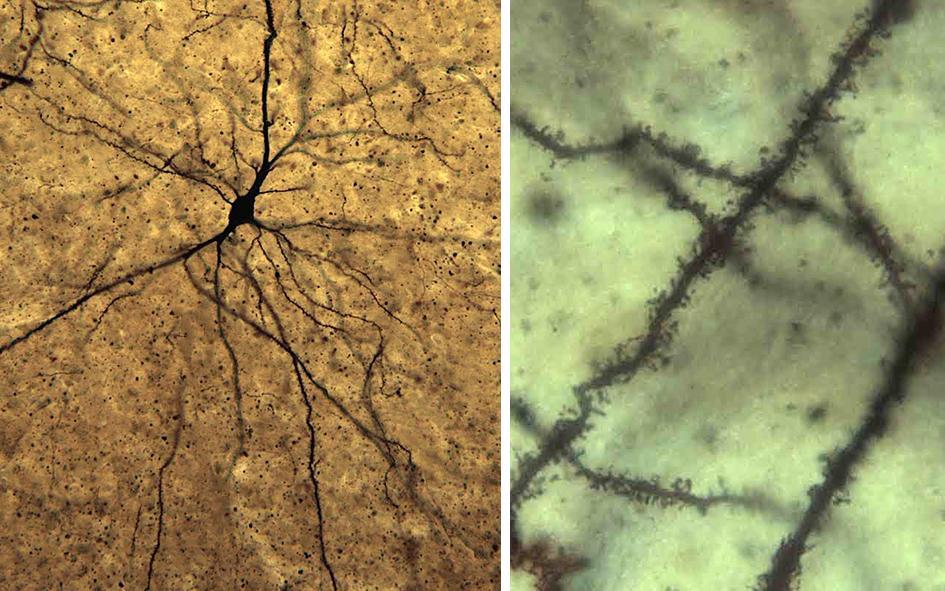
Golgi image: These two photomicrographs show neurons that have undergone Golgi staining – a process that reveals the entire structure of individual neurons, from the cell body and larger dendrites (lower magnification image on the left) through to the tiny dendritic spines where synapses often occur (higher magnification image on the right). These neurons are from the elephant brain, but these images show that the technique works very well in the brains of animals from the African Savannah Ecosystem.
Specific Aim 5: Employing whole-transcriptome analytical methods we plan to investigate hippocampal gene expression by quantifying the mRNA transcript levels (Oldham et al., 2008; Romero et al., 2012; Qureshi and Mehler, 2012; Konopka et al., 2012). Samples containing three subfields from the dorsal and ventral hippocampus (DG, CA3, CA1) will be sampled using biopsy tissue punches. Once in tubes of TRIzol solution, RNA will be extracted from each sample by lysing cells with a TissueRuptor (QIAGEN), isolated using a centrifuge (Eppendorf), and stored for 24 hours in RNAlater (QIAGEN). Extracted RNA will be sent to the Genome Technology Access Center at GWU for cDNA library preparation and next generation sequencing (NGS) of total RNA (both polyadenylated mRNA and small non-coding RNA) on the HiSeq 3000 sequencing system (Illumina). Gene alignment and analyses will be conducted in R (R Development Core Team 2015) using various packages within the BioConductor suite (Huber et al., 2015), including edgeR to identify differential expression between species and weighted gene co-expression network analysis (WGCA) to identify species-specific gene networks, and goseq to test for enrichment of specific gene ontology categories (e.g. neurogenesis, synaptic plasticity) in species-specific genes/networks. Based on these results, genes of the greatest interest will be further investigated using qPCR (as a complementary means to confirm gene expression differences) and cIHC (to identify distribution patterns of the protein products of candidate genes). We expect that candidate genes known to be particularly important for hippocampal plasticity and adult neurogenesis will be differentially expressed in the plains zebra compared to other species, including: doublecortin (DCX), glial fibrillary acid protein (GFAP), brain-derived neurotrophic factor (BDNF), and ephrin-B1 (EFNB1) (Knoth et al., 2010; Waterhouse et al., 2012; Cissé and Checler, 2015).
Specific Aim 6: Using advanced diffusion MRI techniques, we aim to describe and quantify the patterns of connectivity within the neural navigation system of the plains zebra and other species targeted for investigation. By using cutting-edge diffusion MRI tractography methods, the connectivity patterns of the navigational system (using the different component parts identified in histological studies, see objective 1) across species can be identified and compared (cf. Mars et al., 2016a). Using recently developed analytical techniques (Mars et al., 2016b; 2018) it is possible to quantitatively identify which parts of the cortical network differ between plains zebras and phylogenetically related and sympatric species. This allows us to investigate whether the navigational system is differentially connected within the zebra, indicating a different informational flow to and from this system. Similar techniques have successfully compared primate brain organization (Mars et al., 2013; 2018).
